pharma-industry-storage
Patients’ safety right from the start.
Your partner for quality and compliance in pharmaceutical storage.
Safety first: Measuring instruments and all-in-one solutions for pharmaceutical storage
When storing pharmaceuticals, a high degree of compliance awareness is required in order to work safely and in accordance with the standards. This is because the indoor climate in storage rooms has an effect on the efficacy and quality of pharmaceuticals and, thus, on people’s health.
With complete solutions, data loggers and metrological services from Testo, you establish a constant and standard-compliant storage climate:
• Complete solutions (monitoring systems) consisting of sensors, software and services record and document all audit-relevant environmental parameters.
• Measurement technology such as data loggers seamlessly records the relevant environmental parameters.
• Metrology services such as validation, qualification and mapping ensure that measurement technology is used optimally and that your premises and processes comply with current standards. The documentation is also standard-compliant, so you can look forward to the next audit with confidence.
Comprehensive support by Testo enables you to respond to any deviations in good time and in addition, to reliably comply with regulations covering GSP (Good Storage Practice), GDP (Good Distribution Practice) and 21 CFR Part 11.
The complete solutions: Environmental monitoring with testo testo Saveris 1 and testo Saveris 2
Safety, reliability and conformity to standards are important in the storage of pharmaceuticals.
Safety: The measurement technology used must ensure that the quality and integrity of the stored products are not compromised.
Reliability: Environmental parameters must be monitored seamlessly – 24 hours a day. And in the event of limit violations, it is mandatory that an alarm be triggered.
Conformity to standards: Both the storage conditions themselves and the solutions used for monitoring must comply with current standards and guidelines.
The two industry-leading monitoring solutions testo Saveris 1 and testo Saveris 2 provide automated and efficient support in complying with your regulatory requirements.
testo Saveris 1: Fully automated environmental monitoring

The environmental monitoring system records and analyzes your critical environmental data, alerts you immediately if limit values are violated and can help you optimize your processes.
• Holistic system: Sensors, software and services
• Seamless recording and documentation: All audit-relevant environmental parameters in the storage of pharmaceuticals under control at all times
• Standard-compliant: GxP and 21 CFR Part 1
testo Saveris 2: Radio data logger system

• All measurement values in view: Anytime, anywhere and with any internet-enabled device
• Respond promptly: Alarms via SMS or e-mail
• Simply start up: Without software installation – internet and browser are enough.
The services: Validation, qualification and mapping
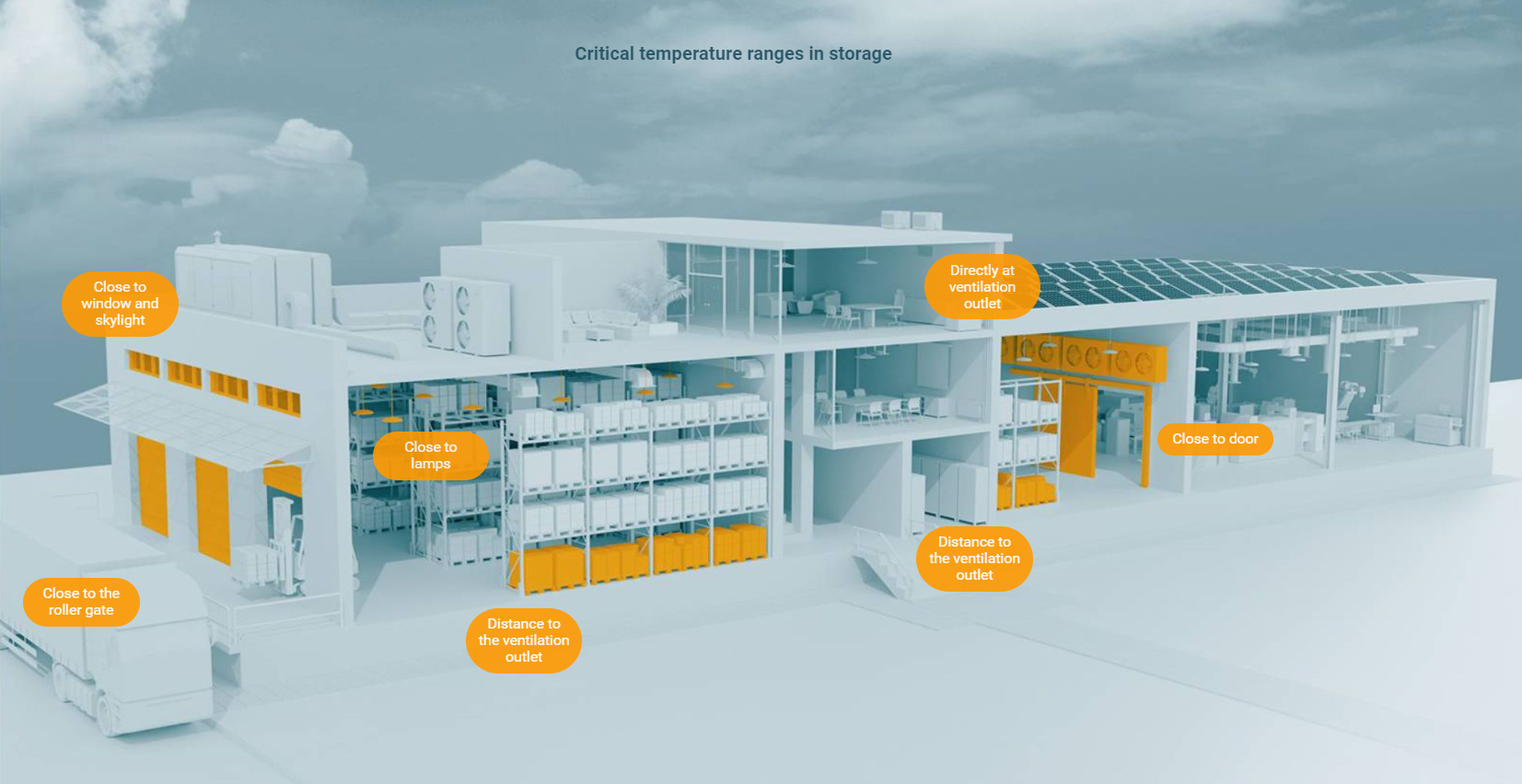
Goods and products that are sensitive to temperatures must be manufactured, stored and transported in qualified facilities, with qualified equipment and using validated processes. Here, the three sub-processes of validation, qualification and mapping are of particular importance. Among other things, critical temperature ranges can be identified and specifically monitored to ensure that the safety of the stored pharmaceuticals is not compromised at any time.
No effort, no gaps, no worries: Qualification and mapping of your storage areas

Validation
The purpose of validation is to ensure that a manufacturer’s expected results can be guaranteed. A validation process takes place in three steps:
• Definition of the expected results and acceptance criteria.
• Verification and documentation that the process delivers the expected result.
• Determination of continuity, repeatability and accuracy of the validated process.

Qualification
Qualification is the process of demonstrating that facilities and equipment are functioning properly.
For example, the qualification of a refrigerator or a high-bay warehouse ensures that all prescribed environmental conditions are met. Products that require refrigeration or are unstable pose particular challenges when it comes to storage.
A mapping (also called temperature or climate mapping) is part of this process step.

Mapping
Temperature mapping identifies the zones of your storage, refrigeration and freezer areas that are within safe parameters for holding your products.
Mapping furthermore determines the performance limits of the air-conditioning systems. It also serves to identify critical control points in the refrigerator or storage area.
This enables you to optimally position the measuring points in your monitoring system and keep an eye on all the key parameters.

We are proud of this:
The objective of the cooperation between Testo and Grieshaber Logistics Group AG was the creation and establishment of an overall GMP concept for a new warehouse in Rheinfelden, Germany.
The data loggers: Three model series for comprehensive monitoring
For decades, data loggers from Testo have been used all over the world wherever precision, reliability and flexibility are required. The different model series cover a wide range of applications. What do our data loggers have in common? They simply offer you more:
• More accuracy: Highly precise from -200 to +1000 °C
• More security: Detailed alarm management and measurement data storage even when the battery is empty
• More reliability: Seamless recording and documentation of up to 2 million measured values with 8 years battery life
• More choice: Extensive probe range in brand quality from series model to custom-made

| Data logger testo 174 |
• Small and compact for monitoring and mapping in warehouses
• For temperature and humidity
• Large display and USB port
• Programming and analysis using free software

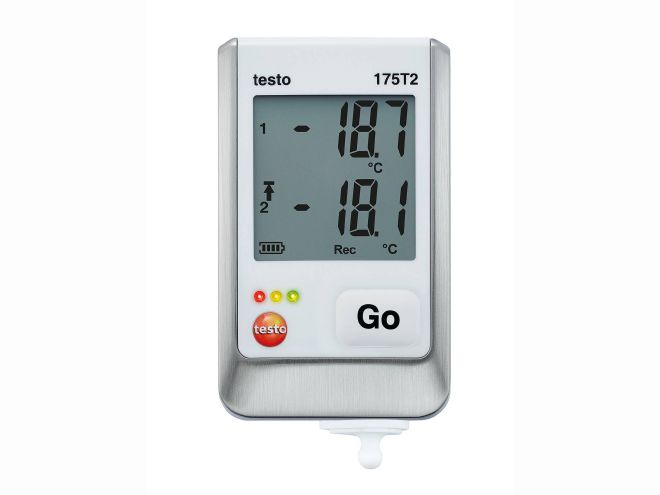
| Data logger testo 175 |
• Comprehensive models with integrated and connectable probes
• For temperature and humidity
• Large display and USB port
• Programming and analysis using free software

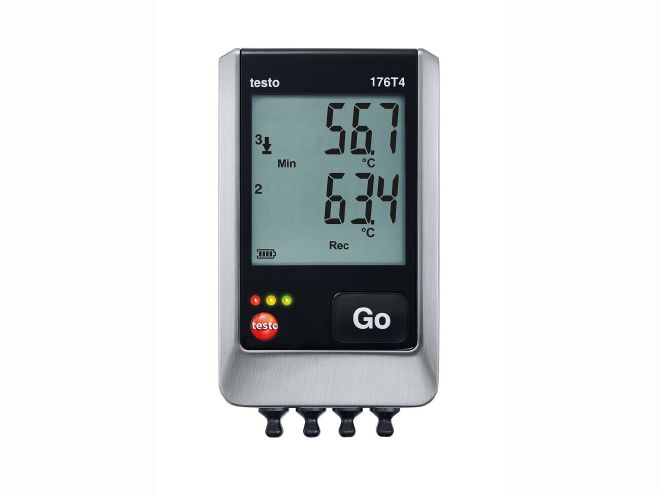
| Data logger testo 176 |
• Extremely robust, specially for use in rough surroundings
• Stores up to 2 million readings
• Up to 8 years battery life
• Programming and analysis using free software